What are seagrasses?
Seagrasses are aquatic flowering plants that form ‘meadows’ in near-shore brackish or marine waters, in temperate and tropical regions. Australia has approximately 51,000 km2 of seagrass meadows, comprising the most diverse array of seagrass species in the world1. Coastal seagrasses are particularly diverse, and can be found in subtidal and intertidal environments2. Seagrass meadows are very productive, support complex food webs and are valued as a habitat and refuge for a number of organisms3.
Changes in seagrass areas indicate major changes in environmental characteristics, and are an important indicator for State of the Environment reporting4. (e.g. Indicator 2.9 in the Estuaries and the Sea volume).

Photo 1. A Zostera meadow (photo supplied courtesy of CSIRO Land and Water).
What causes seagrass areas to change?
- Seagrasses respond to natural variations in light availability, nutrient and trace element (iron5) availability, grazing pressure, disease6, weather patterns, and episodic floods and cyclones1. The dynamic nature of seagrass meadows in response to natural environmental variation complicates the identification of changes caused by humans1.
- Seagrasses have high minimum light requirements (e.g. 4.4 – 29% of surface irradiance)7 because: (i) they have a high respiratory demand to support a large non-photosynthetic biomass (e.g. roots, rhizomes)8; (ii) they lack certain pigments and therefore can utilise only a restricted spectral range9; and (iii) they must regularly oxygenate their root zone to compensate for anoxic sediment10. The most widespread and pervasive cause of seagrass decline is a reduction in available light11. Processes that reduce light penetration to seagrasses include pulsed turbidity events during floods, enhanced suspended sediment loads, and elevated nutrient concentrations12. Phytoplankton and fast-growing macroalgae are also better competitors for light than benthic plants, and their biomass can shade seagrasses during progressive eutrophication (Figure 1)5.
- Water column nitrate enrichment13 and trace metal contamination14 can exert direct toxic effects on some seagrass species. Seagrasses are able to bioaccumulate the trace metals and this can have ramifications for grazers such as dugongs14.
- Seagrasses are susceptible to fishing pressures such as commercial and recreational harvesting of fish and shellfish1. For example, trawling and hauling gear, and boat, vehicular and foot traffic cause direct damage to plants1. The removal of forage or predator species can also have detrimental effects that cascade through food chains1.
- Some introduced marine pests and pathogens have the potential to damage seagrass meadows15.
- Seagrass meadows can change in response to chemical (e.g. salinity and pH), thermal, structural and biological disturbances caused by freshwater extraction1 and changes in water movement due to coastal developments (including ports and marinas). Seagrass meadows may also contract when mangrove areas expand in response to increased suspended sediment loads1. This process is called ‘narrow banding'1. Extraction, filling, dredging and sand mining activities also cause direct damage to seagrass areas1.
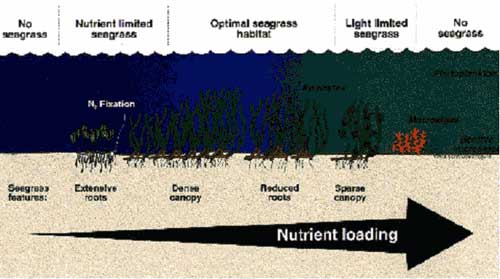
Figure 1. Nutrient loading effects on seagrass systems (From Butler (1999) 1 ). Reproduced with permission of the Fisheries Research and Development Corporation).
The importance of seagrass meadows
Seagrasses are an important link in the “critical chain” of habitats required for sustainable fisheries production15. Seagrasses are extremely productive, have a high biodiversity, and provide nursery habitat for an abundance of fish, crustaceans and molluscs, including many commercially valued species3. With the exception of sea turtles and dugongs which graze directly on seagrasses (Figure 2), most of the carbon fixed by seagrasses enters the food web via detritivores rather than through direct consumption. Seagrasses bind sediment and help to stabilise shorelines against erosion1. The baffling effect of seagrasses on water movement causes the deposition of suspended sediment and organic matter1. Baffling can also impede the mixing process which destabilises water column stratification, thereby influencing the dissolved oxygen status of bottom waters16.
A wide range of nutrient cycling processes occur in coastal seagrass communities, and these vary according to seagrass species and the extent of grazing12. Seagrasses can support high rates of nitrogen fixation17, denitrification18 and sulfate reduction17. Uptake of nutrients by seagrasses (and other benthic plants and algae) can also change the status of sediment from a nutrient source to a nutrient sink19. Seagrass meadows also have the potential to influence physical and chemical parameters in the water column. Dissolved oxygen saturation values commonly exceed 130% in Ruppia meadows in salt lakes, and photosynthetic consumption can lower water column partial pressures of carbon dioxide by ~160 times20.
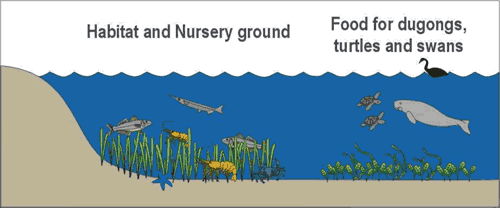
Figure 2. The ecological role of seagrass meadows (From Dennison and Abal (1999)21). Reproduced with permission of Healthy Waterways Library.
Considerations for Measurement and Interpretation
Aerial photography, satellite imagery and systematic towed video surveys can be used to map the extent of seagrass coverage in some coastal waterways, although ground truthing to the genus level by local agencies is advised. High levels of turbidity can complicate seagrass mapping in tide-dominated coastal water waters (e.g. deltas, estuaries and tidal creeks). Chapter 4 of ‘Seagrass in Australia: Strategic Review and Development of an R & D Plan1 reviews existing literature on the monitoring and assessment of seagrasses, and suggests recommendations for future directions. A new book (2001) entitled “Global Seagrass Research Methods” (Eds F.T. Short and R.G. Coles) is also a useful resource. Other usefuls chapters can be found in the recent edition (2007) of Seagrasses: Biology, Ecology and Conservation (Larkum, Anthony W.D.; Orth, Robert J.; Duarte, Carlos M. (Eds.)).
There have been significant advances in the determination of seagrass properties other than coverage (i.e. species composition and biomass) from satellite imagery. Some links to relevant case studies are provided below:
Seagrass species, biomass and %cover maps in Moreton Bay
Detection of Changes in Seagrasses Composition in Wallis Lake, New South Wales.
Case Studies
Integrated field and remote sensing approach to map seagrass cover in Moreton Bay
Moreton Bay is a complex coastal embayment, supporting large seagrass beds, with variations in water clarities evident across the Bay and at different times of the year. Creating accurate, reliable and cost-effective maps of seagrass properties in these environments is a challenging procedure. An integrated approach was used to create a seagrass cover map from multiple data sources collected by natural resource management agencies and volunteer programs2223) (Figure 3). Field survey data such as spot check (direct observation and via video) and photo-transects in combination with, Landsat 5 Thematic Mapper satellite imagery, were collected and analysed by several agencies during July-September 2004. Field data were used to: (1) train seagrass cover mapping algorithms applied to areas of Moreton Bay where the bottom was visible; and (2) to manually digitize seagrass cover for the remaining areas where it was too turbid or deep to see the bottom. The resulting seagrass cover map included the entire Bay. The collaborative and integrative method used serves as a model for integrating activities of multiple government, scientific and volunteer agencies to map and monitor a natural resource.
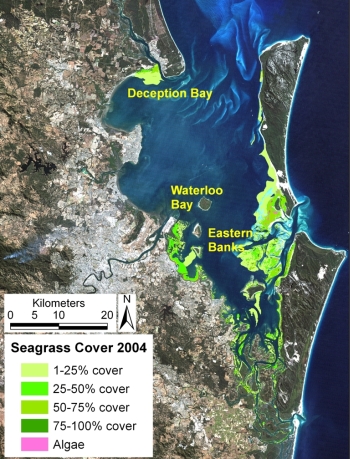
Figure 3. Seagrass cover map of the seagrass in Moreton Bay, Australia, resulting from a combination of field data collected in July 2004 (by natural resource management agencies and volunteer programs) and Landsat TM5 satellite image acquired 8th August 2004 of Moreton Bay, South East Queensland9.
Existing information and data
Interactive Habitat Extent and Distribution Mapping Interface
The National Intertidal/Sub-tidal Benthic Habitat Classification scheme (NISB) habitat classes include: mangroves, saltmarsh, seagrass, macroalgae, coral reef, rock-dominated, sediment-dominated and filter feeders (such as sponges). These habitats occur between the approximate position of the highest astronomical tide mark and the location of the outer limit of the photic benthic zone (usually at the 50 to 70 metre depth contour). High spatial resolution polygons with thematic attributes based on NISB are available in the NRM Reporting module, together with national, state and regional summary maps for each habitat.
A strategic review and development of an R&D plan for Australian seagrasses was commissioned by the Fisheries Research and Development Corporation1 (FRDC) and follows from ‘The Fisheries Habitat Review'15. A document summarising the research is available from theSeagrass Research: Priorities for Seagrass Reasearch in Australia Community-based seagrass monitoring programs, for example Seagrass-Watch, exist in some states. The Ecosystem Health Monitoring Program provides information on the distribution and depth range of seagrasses in Moreton Bay since 1992 and is a useful resource. The influence of environmental factors on seagrass biomass and depth ranges can be explored in the Simple Estuarine Response Model developed by CSIRO. The Department of Environment and Water Resources (Australia) provides guidelines for State of the Environment reporting. In the recent edition (2007) of Seagrasses: Biology, Ecology and Conservation (Larkum, Anthony W.D.; Orth, Robert J.; Duarte, Carlos M. (Eds.)), experts in 26 areas of seagrass biology present their work in sections that relate to taxonomy, anatomy, reproduction, ecology, physiology, fisheries, management, conservation and landscape ecology. There is also a detailed chapter on the remote sensing of seagrass ecosystems.
Coastal Remote Sensing Toolkit
Maps of the coastal environment are needed for inventory, monitoring and management. Remote sensing instruments on various platforms (boat, aircraft and satellite) and processing techniques can be used to collect appropriate image data and convert them into maps of relevant properties. Remote sensing provides an opportunity to produce maps that can be: accurate, reliable and cost-effective. There is a continually expanding array of boat, airborne and satellite based image types and processing approaches to choose from for mapping and monitoring coastal environments. A web based instructional toolkit has been created to inform managers, scientists and technicians working in coastal marine environments how images collected from satellites and aircraft can be used to map and monitor changes to indicators of coastal ecosystem health24.
Coastal Habitat Resources Information System (CHRIS)
Queensland Government – Department of Agriculture & Fisheries (CHRIS) has an interactive map facility that enables users to make detailed vegetation maps for the Queensland coast (see coastal wetland layers) in including various densities of seagrass beds (Figure 4).
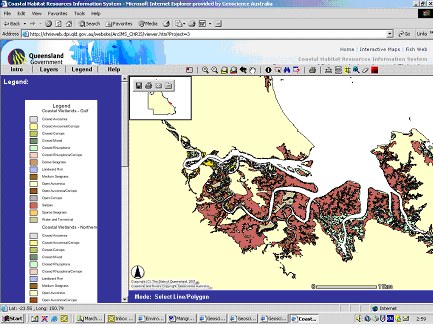
Figure 4. Screen dump showing detailed vegetation map for tidal creeks near the Fitzroy Estuary and Keppel Bay based on CHRIS.
More information on habitat removal/disturbance.
Contributors
Vittorio Brando, CSIRO Land and Water
Ben Longstaff, University of Queensland
Stuart Phinn, University of Queensland, Centre for Remote Sensing and Spatial Information Science
Chris Roelfsema, University of Queensland, Centre for Remote Sensing and Spatial Information Science
- Butler, A.J. 1999. Seagrass in Australia: Strategic Review and Development of an R&D Plan, FRDC Project 98/223. ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩
- Lee Long, W.J., Coles, R.G., and McKenzie, L.J. 2000. Issues for seagrass conservation management in Queensland. Pacific Conservation Biology 5, 321-328. ↩
- Loneragan, N.R., Kenyon, R.A., Haywood, M.D.E., and Staples, D.J. 1994. Population dynamics of juvenile tiger prawns (Penaeus esculentus and P. semisulcatus) in seagrass habitats of the western Gulf of Carpentaria, Australia. Marine Biology 119, 133-143. ↩ ↩
- Ward, T., Butler, E. and Hill, B. 1998. Environmental Indicators for National State of the Environment Reporting, Estuaries and the Sea, Commonwealth of Australia, pp. 81. ↩
- Duarte, C.M. 1995. Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41, 87-112. ↩ ↩
- Giesen, W.B.J.T. Van Katwijk, M.M. and Den Hartog, C. (1990b) Eelgrass condition and turbidity in the Dutch Wadden Sea. Aquatic Botany 37 (1):71-86. ↩
- Dennison, W.C., Orth, K.A., Moore, R.J., Stevenson, J.C., Carter, V., Kollar, S., Bergstrom, P.W. and Batiuk, R.A. 1993. Assessing water quality with submersed aquatic vegetation. Bioscience 43:86-94. ↩
- Fourqurean, J.W. and Zieman, J.C. (1991) Photosynthesis, respiration and whole plant carbon budget of the seagrass Thalassia testudinum. Marine Ecology Progress Series 69 (1-2):161-170. ↩
- Frost-Christensen, H. and Sand Jensen, K. (1992) The quantum efficiency of photosynthesis in macroalgae and submerged angiosperms. Oecologia 91 (3):377-384. ↩ ↩
- Terrados, J., Duarte, C.M., Kamp Nielsen, L., Agawin, N.S.R., Gacia, E., Lacap, D., Fortes, M.D., Borum, J., Lubanski, M. and Greve, T. (1999) Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquatic Botany 65:175-197. ↩
- Walker, D.I. and McComb, A.J. 1992. Seagrass degradation in Australian Coastal Waters. Marine Pollution Bulletin 25, 191-195. ↩
- Carruthers, T.J.B., Dennison, W.C., Longstaff, B., Waycott, M., McKenzie, L.J., Lee Long, W.J. in prep. Seagrass habitats of north east Australia: models of key processes and controls. ↩ ↩
- Burkholder, J.M., Mason, K.M., Glasgow, H.B. 1992. Water-column nitrate enrichment promotes decline of eelgrass Zostera marina: evidence from seasonal mesocosm experiments. Marine Ecology Progress Series 81, 163-178. ↩
- Prange, J.A. and Dennison, W.C. 2000. Physiological responses of five seagrass species to trace metals. Marine Pollution Bulletin 41(7-12), 327-336. ↩ ↩
- Cappo, M., Alongi, D.M., Williams, D. and Duke, N. 1995. A Review and Synthesis of Australian Fisheries Habitat Research: Major threats, issues and gaps in knowledge of coastal and marine fisheries habitats. Fisheries Research and Development Corporation. ↩ ↩ ↩
- McLean, E. 2002. Personal Observation, Coasts and Estuaries Section, LWC Sydney South Coast Region. ↩
- Hansen, J.W., Udy, J.W., Perry, C.J., Dennison, W.C., and Lomstein, B.A. 2000. Effect of the seagrass Zostera capricorni on sediment microbial processes. Marine Ecology Progress Series 199, 83-96. ↩ ↩
- Harris, G.P. 1999. Comparison of the biogeochemistry of lakes and estuaries: ecosystem processes, functional groups, hysteresis effects and interaction between macro- and microbiology. Marine and Freshwater Research 50, 791-811. See also references found within. ↩
- Eyre, B.D. and Ferguson, A.J.P. 2002. Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic micro-algae and macroalgae dominated warm-temperate Australian Lagoons. Marine Ecology Progress Series 229, 43-59. ↩
- Radke, L.C. 2000. Solute divides and chemical facies in southeastern Australian salt lakes and the response of ostracods in time (Holocene) and space. Ph.D thesis, Department of Geology, The Australian National University, pp. 232. ↩
- Dennison, W.C. and Abal, E.G. 1999. Moreton Bay Study: A Scientific Basis for the Healthy Waterways Campaign, South East Queensland Water Quality Management Strategy, pp. 245. ↩
- Phinn, S., Roelfsema, C., Dekker, A. , Brando, V., Anstee, J.and Daniel, P. (2006) Remote Sensing for Coastal Ecosystem Indicators Assessment & Monitoring (SR). SR 30.1 Final Report: Maps, techniques and error assessment for seagrass benthic habitat in Moreton Bay. Coastal CRC Technical Report No 76, 115 pp. CRC for Coastal Zone, Estuary and Waterway Management, Brisbane. ↩
- EHMP(2006) Ecosystem Health Monitoring Program 2004-2005 Annual Technical Report. Moreton Bay Waterways and Catchments Partnership, Brisbane (166 ↩
- Coastal Remote Sensing Toolkit(2006). Coastal CRC and Centre for Remote Sensing and Spatial Information Science, The University of Queensland. ↩